Legislation relating to implantation
From the 24th February 2015 anyone wishing to implant microchips in dogs in England may only do so if:
- They are a veterinary surgeon or a veterinary nurse acting under the direction of a veterinary surgeon;
- They are a student of veterinary surgery or a student veterinary nurse and in either case acting under the direction of a veterinary surgeon;
- They have been satisfactorily assessed on a training course approved by the Secretary of State for that purpose;
- Before these Regulations come into force they received training on implantation which included practical experience of implanting a microchip.
Provisions 1 and 2 also apply to anyone wishing to implant microchips in cats in England from 10th June 2024. In addition, cats can be microchipped by:
- someone who has been satisfactorily assessed on a training course approved by the Secretary of State on or after 16 May 2023 for implanting a microchip in a cat
- someone who has been trained in implantation which included practical experience of implanting a microchip in a cat before 16 May 2023
Similar provisions for the implantation of dogs are included in the regulations that apply in Wales and Scotland, although the Welsh Regulations indicate that the ‘grandfather’ rights of provision 4 above ceased 2 years after the regulations came into force (i.e. April 2018).
In Northern Ireland, a dog must be microchipped by a ‘competent person’, defined as a veterinary surgeon or person who has received instruction on how to implant a microchip.
Once these regulations are in force, anyone implanting a microchip who is not covered by groups 1-4 above is punishable on conviction by a fine of up to level 2 on the standard scale (currently £500).
Lantra has developed a Level 3 Award in Performing Microchip Implantation in Animals. The Lantra qualification covers:
- Knowledge and understanding of current legislation and safety requirements
- Knowledge and understanding of the use of microchips in animals
- How to safely handle and restrain animals
- How to select and prepare the animal for microchipping
- How to safely perform microchip implantation
- How to carry out post-implantation procedures
As far as BSAVA is aware, microchipping courses are available from the following providers:
The Microchip Trade Association
Peddymark
VetSkill
iPet Network
Some of these courses may be approved by Lantra. If individuals are interested in participating in a course specifically approved by Lantra, they should contact Lantra or the course provider to verify this.
There is currently no list of those qualified to implant microchips and it is not the responsibility of the veterinary surgeon to check on the qualification of the implanter of any animal that has already been microchipped. However, it is now a legal requirement in England, Wales and Scotland to report adverse events (reaction, migration, failure) to Defra. For further information see the Adverse reactions section.
It should be noted that definition of adverse reactions in the regulations includes ‘any suffering or pathology on the part of a dog which is caused, or appears to be caused, by a microchip implanted in the dog’ and therefore mis-implantation of a microchip causing suffering or pathology should also be reported.
These definitions and the requirement to report adverse events also apply to owned cats in England.
Form of microchip
All dogs and puppies requiring implanting after 6th April 2016 need to be implanted with an FDX-B microchip conforming to ISO standard 11784, operating at 134.2 KHz and programmed with a unique number starting with a manufacturer code*.
All dogs and puppies over 8 weeks of age by 6th April 2016 that have already been microchipped but have a microchip other than the type specified above, will need to be re- implanted with a compliant microchip conforming to the above requirements. Non-conforming implant types include FDX-A microchips which all have 10 digit numbers, encrypted microchips, or any other not able to be read by an ISO-compliant transceiver reading at 134.2KHz.
For dogs that have been imported, implantation with an appropriate microchip (if not already in place) and registration must occur within 30 days of arrival unless the keeper has an exemption certificate for the dog. If the dog is intended to be transferred to another keeper the dog must be microchipped with a compliant microchip before it is transferred.
Imported dogs already carrying a microchip that conforms to ISO 11784 and ISO 11785 where the number starts with a country code* comply with the UK regulations and do not have to be implanted with a second microchip. However, they do need to be registered on an appropriate database to comply with the regulations.
* All manufacturer codes are three digits long and start with a 9 e.g.: 958000010123456
Country codes are also three digits long but the first digit is never a 9.
These regulations also apply to owned cats over the age of 20 weeks in England from 10th June 2024.
Implantation procedure
In the UK, implantation of a microchip by the subcutaneous route is not considered to be an act of veterinary surgery. However, under the following circumstances even subcutaneous implantation may be classed as an act of veterinary surgery and therefore will need to be carried out by, or under the direction of, a veterinary surgeon:
- Where repair or closure of the entry site is required;
- Where sedation and analgesia are required to facilitate safe implantation;
- If there is special risk to the health or welfare of the animal.
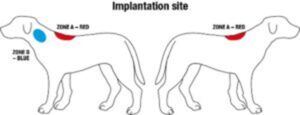
Although the regulations do not specify the site of implantation it is recommended that microchips are implanted at the standard implantation site to minimise the risk of migration and to maximise the chance that the microchip is found on scanning.
There are two standardized injection sites for microchip implantation in dogs and cats which are described in ISO 15639-1.
In the UK and the Republic of Ireland the standard implantation site for dogs and cats is subcutaneous on the dorsal midline so that the transponder lies halfway between the anterior and posterior edge of the scapulae after implantation (the middle of Zone A – coloured in red below). For the purposes of scanning, it should be noted that the standard implantation site for both dogs and cats in continental Europe is subcutaneously on the left side of the body in the cranial third of the neck between the ear and shoulder (Zone B – coloured in blue).